Trusted by the NIH and research teams across the country to engage, educate, and gather data from more than 400,000 research participants.
Request a PilotScreen and Enroll Participants Digitally
Allow prospective participants to easily complete your required screening, enrollment processes, and eConsent to join your study – on mobile web, over the phone, or via mHealth mobile app.
Empower study staff to assist participants with enrollment by using a tablet or other mobile device at clinic visits, recruitment events, or any other interaction.
Request a Pilot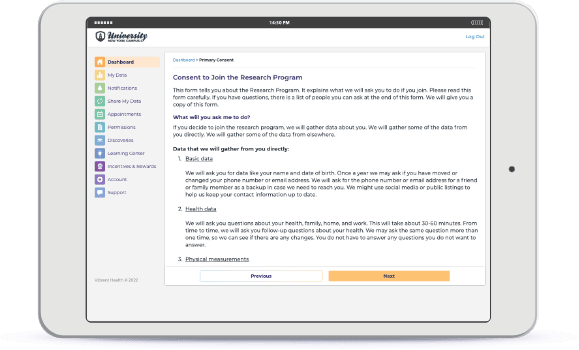
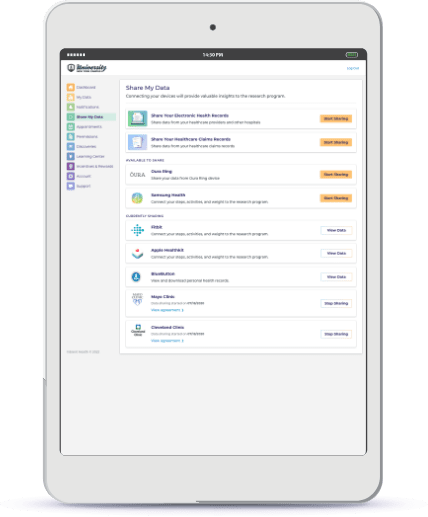
Easily Connect Wearables, EHR Data, and More
Enable participants to complete surveys, Ecological Momentary Assessments, demographics, and other data easily through mobile or web applications.
Simplify participant consent process to connect EHRs. Streamline the collection of participant-generated health data by providing self-service connection to participants’ wearables and medical devices.
Automate patient-generated health data collection from Fitbit, Apple Health, Apple Watch, Google Fit, Samsung Health, and others. Make connection self-service through a configurable guided journey to help participants grant access.
Capture Surveys and EMAs
Reduce the time and potential financial commitment to participate in your research through Participant Experience Manager or your own mobile app.
Increase survey completions for those who have reduced digital access or comfort with built-in computer-assisted telephone interviews (CATI).
Provide your study staff access to only what they need to deliver scripted interviews and accurately capture every answer and datapoint.
Request a Pilot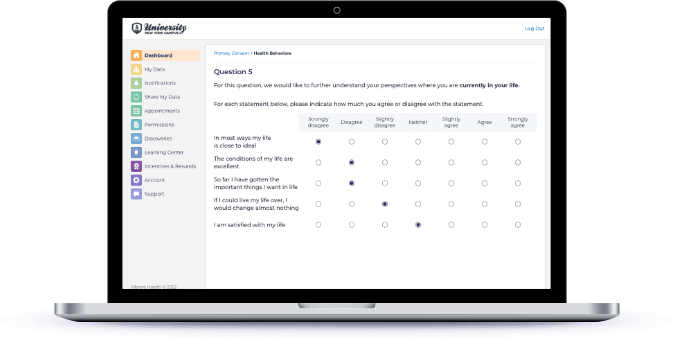
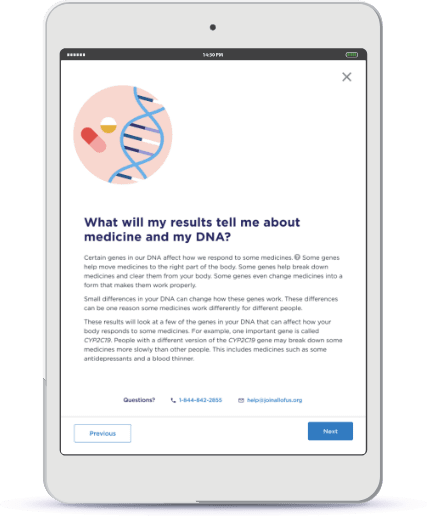
Combine Genomics with Participant Data
Genomics/multi-omics data vastly enhances and accelerates biomedical research, personalized medicine and drug development especially when combined with phenotypic data, environmental data and other contextual data.
Simplify genomic consent and compliance with Participant Experience Manager. Allow participants to request and return specimen collection kits through the portal, and then return results to participants while maintaining privacy and keeping data secure.
Interactive, Informed eConsent
Configure and deploy a seamless eConsent experience, so participants can add their medical data from EHRs and claims.
Confirm diagnoses, understand access to care and medications, and remain compliant and preserve privacy. Recruit participants across state lines while complying with all federal and individual state guidelines for receiving informed consent from participants.
Eliminate the risk of understanding each state’s requirements for eConsent, and meet all IRB, compliance, and regulatory requirements.
Request a Pilot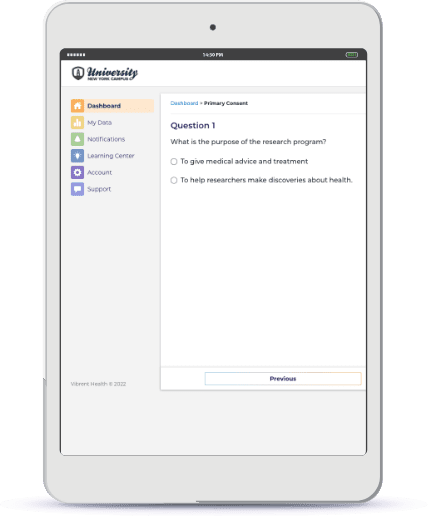
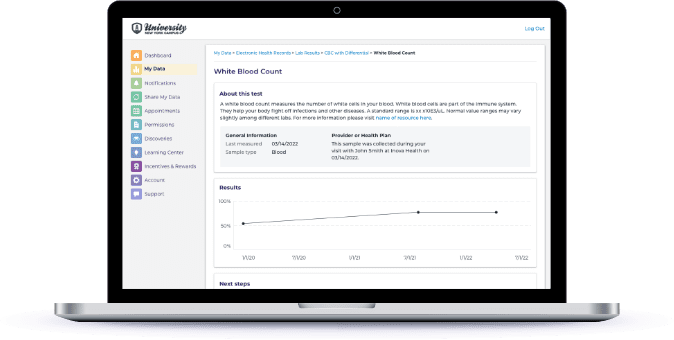
Provide Return of Results and Dynamic Feedback
Give participants access to the data collected about them along with insights into how they compare with others in their cohort.
Transform collected data into eye-opening insights about participants’ health including genomics. Improve long term engagement and retention with dynamic return of value in a personalized way – at scale.
Show participants all of the data they’ve shared with the program to date.
Educate Participants about Their Health
Communicate specific health or research information to the right participants, along with educational content. Automate communication in ways that reduce the burden on clinical or study resources.
Keep engagement high with personal health information and content delivered in a secure experience with privacy, security, and level-of-care requirements at every touchpoint.
And if some participants have preferences about what they learn in your study, empower them to be in control of what they learn about their health.
Request a Pilot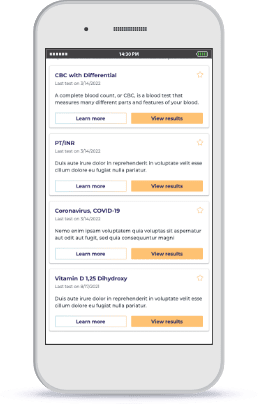
Build Trust at Each Step of the Participant Experience
Configure dynamic and targeted messaging, imagery, colors, and navigation to build trust at every interaction.
Meet the needs of your technology-savvy participants as easily as you bridge the digital divide for those with less access to technology.
Whether it’s a mobile app, low broadband web experience, SMS, phone support, or direct mail, your research methods leave no one behind.
Proven at scale with the NIH’s All of Us program means your participant’s experience has been highly validated.
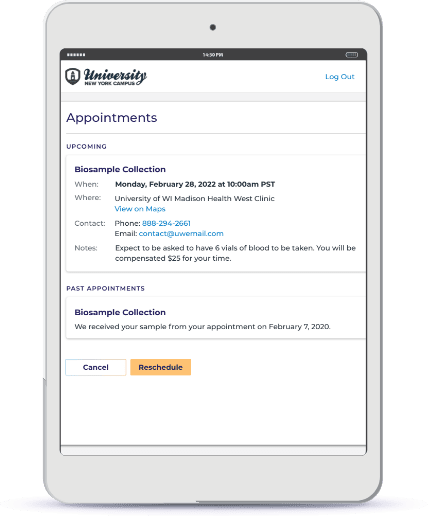
Collect Biospecimen Remotely Using Kits from Different Providers
If you need biological samples in order to finish screening participants, provide self-service for participants to order at-home sample collection kits (genomic, multi-omics, blood, saliva, and more).
Sync the processed data from the biological specimens collected at-home that give insight into someone’s genetic profile and microbiome.
Request a Pilot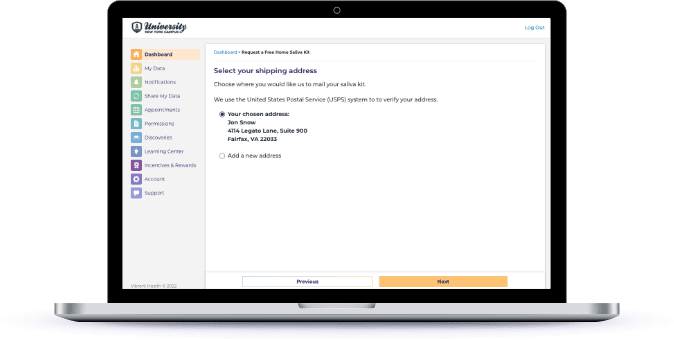

White Glove Service
Our implementation services include meticulous attention to detail to ensure a successful implementation of your study. You focus on the research, and we’ll make sure you can perform it.
Services include:
-
On-demand learning center
-
Individualized training
-
Dedicated support
-
Dedicated project manager
-
IRB submission support
-
eConsent design
-
EHR setup
-
Remote sampling kits
-
Wearable or medical device technologies
-
Recruitment and engagement strategies
Better Data Collection and Better Participant Engagement
with a Suite Built for Research.
Tablet and Mobile Enrollment
Tablet-based and mobile recruitment options for assisted enrollment, Use these tools at recruitment events, in-clinic visits, or any other personal interaction.
eConsent
Turn your paper-based consent into a virtual, interactive experience that informs your participants of the value of participating in research, accommodates multiple learning styles, and makes version control and documentation easy at a fifth grade reading level.
EHR and Data Harmonization
Plug-and-play EHR integrations using HL7/FHIR interoperability standards for participants to access medical records and share data with researchers.
Computer-Assisted Telephone Interview
Enable study staff to complete survey research via telephone call with a fully secure system-guided script.
Dynamic Feedback
Targeted content provides you with a bi-directional model where data, information and knowledge is ethically returned back to participants in a personalized and meaningful way.
Participant Mobile Apps
Generate novel datasets with mobile and web apps, wearables, EHR integrations and connected medical devices. Return value, insights and health promotion to your research participants.