How My Healthy Maryland Uses Vibrent Health to Power eConsent, Recruitment, Data Collection, and Participant Engagement for Its 250,000 Participant Precision Health Study
Background
Precision Health is a medical model that takes into account a patient’s genes, clinical and biomarker information, environment and lifestyle to individualize medical decisions, treatments, and practices. Unfortunately, for Precision Health studies to validate utility requires the collection of data from very large numbers of research participants. In genetics, new technologies like high-throughput genotyping, DNA sequencing, and exome sequencing have shown promise and, in some cases, become the standard of care for rare conditions.

Most genetic studies and discoveries, however, have been made in predominantly Caucasian populations of European origin. This presents a critical gap in our knowledge and understanding of the genetic architecture of health and disease in more diverse populations, and the My Healthy Maryland Precision Medicine Health Initiative aims to help bridge the diversity gap to narrow health care disparities in Maryland and beyond.
Scientific Aims
The primary aim of the My Healthy Maryland Precision Health Initiative is to recruit a large diverse longitudinal cohort of research participants across Maryland for health-related basic, clinical and translational research, and the implementation of evidence-based precision health into the health care systems of the State of Maryland.
Cohort
Large numbers of participants are necessary in order to achieve the research aims of Precision Health studies like My Healthy Maryland. The My Healthy Maryland team initiated the study with a feasibility phase in which they aim to recruit 5,000-10,000 participants, which will be followed by a scale-up phase to as many as 250,000 participants over the next 5-10 years, according to the eligibility criteria:
- Ages 18 and older
- All races
- Maryland resident at the time of enrollment
With a focus on Maryland residents, My Healthy Maryland aims to establish a remarkably diverse dataset, particularly because of the ethnic and racial diversity of the state:
- 51.1% Non-Hispanic White
- 30.9% Non-Hispanic Black
- 10.6% Hispanic
- 7.1% Non-Hispanic Asian or Pacific Islander
- 0.3% Non-Hispanic American Indian
My Healthy Maryland Precision Health Initiative
Approach and Methods for Assessment and Data Collection:
The study uses the following methods of data collection:
Participant Portal
All interested participants can create an account for themselves on the participant portal for My Healthy Maryland Precision Health Initiative. This portal, available as a web or mobile application, runs on Vibrent’s Participant Experience Manager, and provides participants access to informed consent, data collection, their user account details, genomic results, and more information. The portal is one of the primary means for the My Healthy Maryland study to collect data.
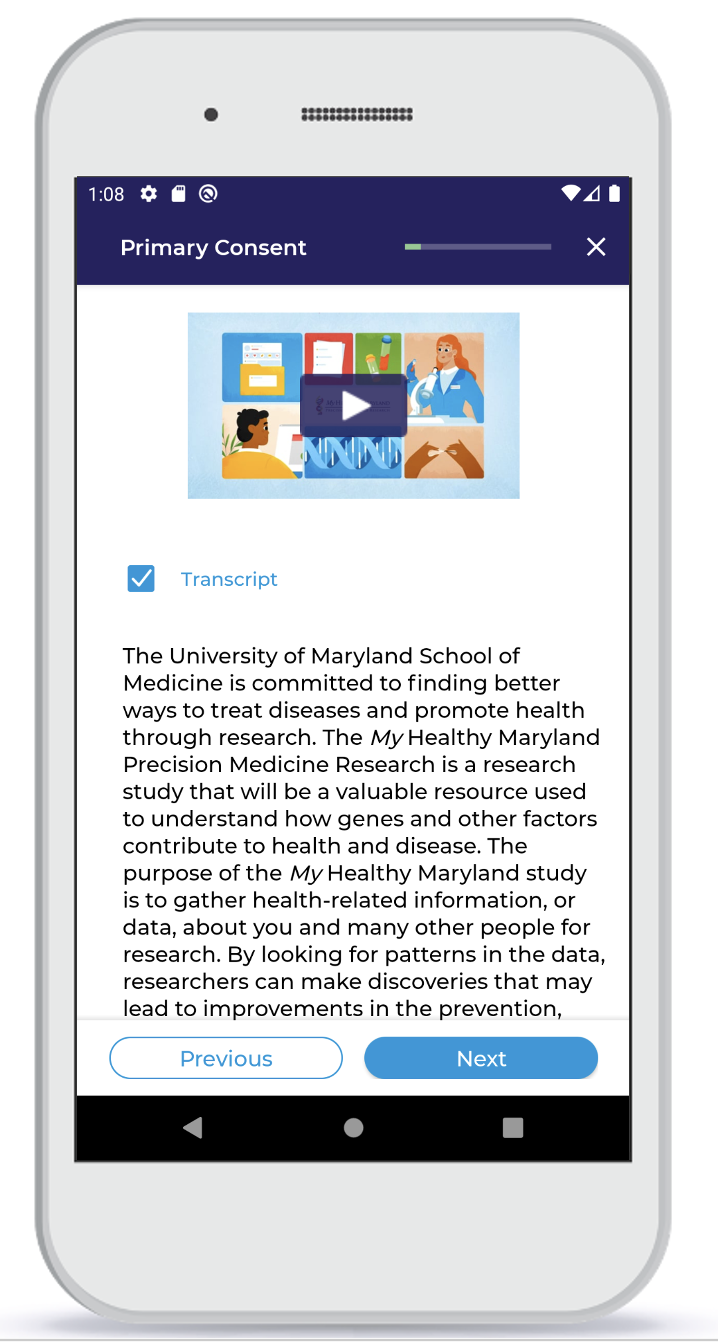
Informed eConsent
The digital informed consent process, as a part of the participant portal, incorporates a series of text screens and videos to educate participants about participating. Agreement to participate is documented with digital signatures and participants are emailed a copy of the IRB approved pdf consent form with their added signatures.
Electronic Health Record Data
The Initiative collects participant electronic health record data (EHR) and links them to survey and biospecimen data for use in a broad range of research. EConsent for this data collection is also acquired through the participant portal, running on Vibrent’s Participant Experience Manager. Data types collected from the EHR include:
- Demographics
- Clinical encounters
- Diagnoses
- Medications
- Laboratory tests
- Anthropometrics
- Procedures
- Vital signs
Participant-Generated Data & Surveys
The study obtains extensive health information from participants through surveys, also run through the participant portal, including:
Electronic Health Record Data
- Basics
- Overall Health
- Personal Medical History
- Family Medical History
- Lifestyle
- Demographics
- Clinical encounters
- Diagnoses
- Medications
- Laboratory tests
- Anthropometrics
- Procedures
- Vital signs
Biospecimen Sample Collection
Biological specimens are obtained from consented participants using:
- Buccal swabs, delivered by mail. These are requested through Participant Experience Manager and mailed with instructions for use at home along with return postage. Up to four swabs are collected from each participant.
- Biospecimens collected from participants under other IRB-approved studies including blood samples, saliva samples, buccal swab samples or extracted DNA
- Residual samples from clinical blood collection
Recruitment and Engagement
To achieve the broad recruitment goals for this study, the My Healthy Maryland study team performs outreach to potential participants using a variety of advertising and communication methods including targeted recruitment, web advertisements, social media, community events and press coverage.
Biospecimen Sample Collection
For all methods of recruitment, interested individuals are directed to the study website, built through Vibrent Research Cloud. The website includes introductory information about the study, FAQs, and links to the study consent form.
Targeted recruitment
Study staff can contact participants who are enrolled in other UM IRB-approved protocols and have agreed to re-contact by email/letter to offer participation.
Email and SMS recruitment
Study staff reach potential participants through personalized email and SMS messages to existing lists of contacts. Potential participants can select their preferred method of communication.
Community events
During community outreach events or other locations, study staff use enrollment and interest forms through Research Cloud to capture information about an interested potential participant. Interested individuals can also download the mobile app or navigate to the web application on their own mobile device or use an on-site kiosk or tablet on which they can review the audiovisual content and provide e-consent.
Participant engagement
Participants are engaged on an ongoing basis by receiving communications via email, push notifications or SMS messaging, including notices of completion of activities (e,g. consent, specimen collection), reminders that action is needed or availability of new surveys, and when errors or problems occur (e.g. locked out of account, forgot password).
Participant portal
In addition to outbound messages, participants are engaged through the participant portal, run on Vibrent’s Participant Experience Manager. Participants can access the portal through the web or mobile application, and gain access to information about their participation, including:
- Genomics results
- Personalized educational content
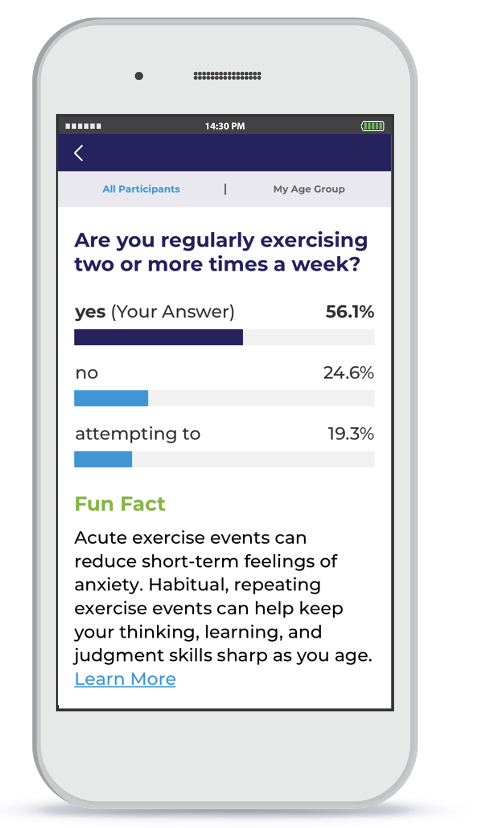
- Quick polls, which allow participants to answer a question to see how their responses compare to aggregated responses from the cohort
- Notifications of actions needed, or new updates
- Personal account details
study principal investigator Stephen Davis, MBBS, FRCP, FACE, MACP, Theodore E. Woodward Professor and Chair of the Department of Medicine at UMSOM, and Director, Institute for Clinical and Translational Research and Vice President of Clinical Translational Science at the University of Maryland, Baltimore.
Research Impact:
The large-scale recruitment of the My Healthy Maryland Precision Health Initiative participants
and their engagement for longitudinal follow up will provide an unprecedented opportunity for researchers to better understand the human genomic variation and its relationship to disease and treatment.
The data collected will be representative of the diversity of Maryland’s population integrating clinical phenomic, genomic and other -omic sciences with state-of-the art big data analytics, informatics, and artificial intelligence to advance basic discovery, epidemiologic, translational, and clinical and implementation research in precision health, to improve the health of Marylanders and the value of healthcare in Maryland and beyond.
Participants can configure their communication preferences to receive updates via SMS or email. Communications may include when their return of results is ready, and if they need to complete additional surveys. They can also change their contact details to ensure that they receive these communications.


